| [E-mail] | |||
CO2 & Water HardnessThough this section is primarily for plantkeepers, it is also the place to find related info, such as Cichlid salt recipes! You might also check the general chemistry section for related information. |
For a quick jumpstart on CO2, first read this intro by George Booth.
Before delving into CO2 proper (and alienating the rest of the aquaria audience), here are a few articles about buffering:
The CO2 content of your aquarium is important if you are growing plants (though check out a discussion on which plants need supplemental CO2, and which may actually do worse with it!). But CO2 is also of interest to the general aquarist because it is intimately related to the water's carbonate hardness (KH) and pH; dissolved carbonates will raise both KH and pH, while addition of CO2 will lower pH. Here are some charts showing the relationship between CO2, pH and KH, along with a discussion of the actual chemical reactions. You can read CO2 content indirectly by from the combined pH and KH readings, from a good test kit, or try this simple metering method.
A few plants are able to take in carbon directly from dissolved calcium carbonate (a procedure called biogenic decalcification by some). This procedure in turn may cause your pH to rise.
You can easily make your own
carbonate buffer from common chemicals.
You can use Calcium Chloride to increase
GH without changing the alkalinity and pH.
Some suggest you can use hydrochloric acid
in extreme moderation to reduce the buffering capacity of your water (this
article also discusses an empirical result of CO2 content vs. KH).
First off, some disasters that can result from mishandling compressed cylinders... be careful!
You can buy a regulator at a brewers supply, welders supply, or beverage supply house. George Booth suggested preset regulators as a cheap alternative to the usual adjustable ones.
Here are sources and other info for needle valves and solenoids, the most popular being the ARO NO-1 and the Nupro S Series.
If you have a lot of money and/or are very picky, you might want to use a pH controller to regulate the flow of CO2. Jim Hurley was one of the first on the net to build a complete automatic system including an electronic pH controller. (See comments from other folks who've tried to build it, including Sherman Lowell, who expounds and improves on the design on his website.) And Gary Bishop describes an inexpensive alternative controller for such a system, using a photocell instead of pH probe.
For measuring the injection rate, you can build a bubble counter. For measuring CO2 level in the aquarium, buy or build an indicator.
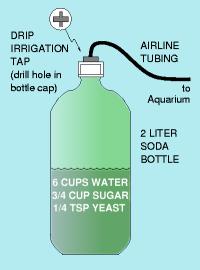
Though the gas cylinder and the yeast bottle are the most popular sources, there are products coming out on the market every year using alternate means of producing the gas.
Here are several experiences with the Tetra CO2 ``bell'' system, which uses compressed gas but requires you to manually turn the tank on and off. Another ``alternative'' setup is based on CO2 cartridges, similar to those used in pellet guns. Here's some (not good) reviews of the Ceomat and bioplast systems, which use chemical reactions. Electrolysis (Carbo-Plus, etc) uses a carbon rod to produce H2 and CO2 gas. One person even theorized using toxic chemicals to generate it. Soda/Seltzer water has been considered by some, though it is impractical and extraordinarily expensive. Finally, there is always human respiration as a means of producing CO2.
Once you have chosen your CO2 source, you must get it to dissolve into the aquarium water.
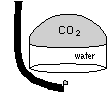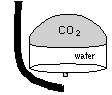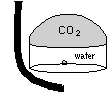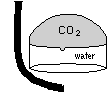
| The easiest, if not the most esthetically pleasing, method is to trap the gas in an upturned cup or ``bell''. While trapped, the gas eventually diffuses into the water |
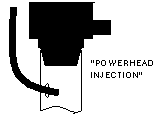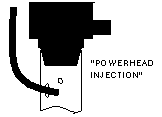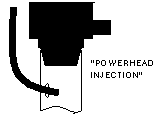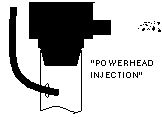
| Another simple approach is to run the CO2 line into the intake of a powerhead of canister filter. The impeller helps break up and dissolve the bubbles. |
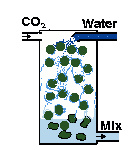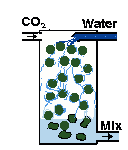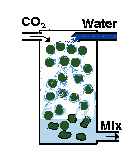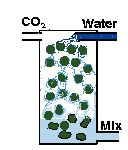
| You can build a gas reactor, which forces the CO2 to quickly mix with rapidly moving water in a small chamber. I (EO) wrote an illustrated article with construction details. So have other people. |
| Relatively new on the scene are ceramic/glass diffusers, available from ADA or Eheim. This method replaces both the reactor and metering valve entirely; The diffuser passes pressurized gas as tiny bubbles through the glass. There have been debates over a possible end-of-tank dump effect if you are not also using a good needle valve to regulate flow. |
Finally, here are some more diffuser and reactor postings for more info. Nadeem Faiz drew up a nice illustrated diagram showing a full yeast generator, bubble counter, and bell diffuser. Incidentally, you might want to look at what kind of hose and valves you use to transport the CO2 to the tank. Cheap rubber check valves are known to disintegrate quickly from the carbonic acid!
|
|
[E-mail] | ||